Lecture summaries for Spring 2020
Back to Kanat's home page, the class page, or send e-mail to Leslie Kanat
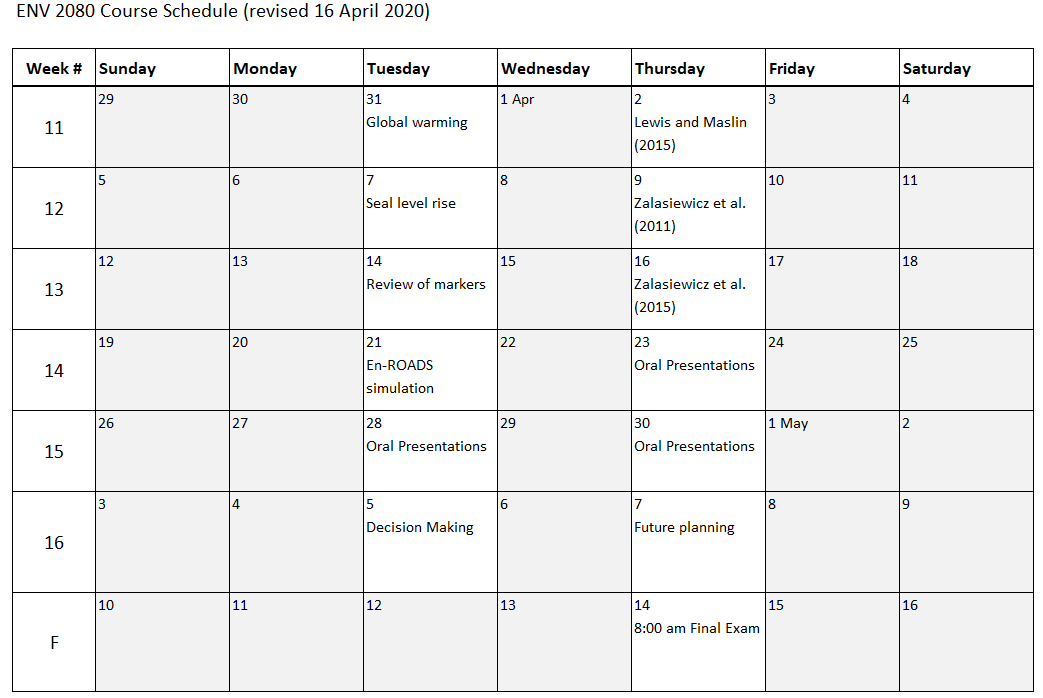
Click on a week for the lecture summaries:
Block the text you want to print, then
click: file > print > selection > print.
The first presentation for this course will be held on Tuesday, 21 January 2020 and start at 10:00 a.m.; we will meet in Bentley 308. The first meeting of the class ends early because of the Welcome Back Celebration that starts at 11:00 a.m. in Dibden.
Textbooks are not required for this course, yet there will be sufficient literature available online.
Student presentations will be assessed for the NVU Graduation Oral Communication Standard. The PowerPoint slides associated with the presentations must incorporate the Assertion-Evidence model (see the following two sites about the design of PowerPoint slides):
Add the Printfriendly extension to the Chrome browser on your computer if you want to print web pages. This add-on removes extraneous advertisements, cleans up the page, and makes the document much easier to read.
NVUJ offers MS Office 2019 for a PC or Mac, for free, click here to get the software.For additional background information about the Anthropocene, I suggest listening to the TED Radio Hour on National Public Radio, Anthropocene, at http://one.npr.org/?sharedMediaId=495657272:496016953
Biello, David (2016). You Have Been Living in a New Geologic Time All Along. Retrieved on 27 November 2016 from http://ideas.ted.com/you-have-been-living-in-a-new-geologic-time-all-along/
Come to class prepared to discuss the paper; take notes and print out the paper (or bring a laptop).
Crutzen, P. J. and E. F. Stoermer, 2000. The Anthropocene in The Global Change Newsletter, v. 41, p. 17-18.
[Introduction to the Anthropocene; moderate read; often cited; informative. It is this paper that is often referred to by others as one of the original source papers on the Anthropocene.]Come to class prepared to discuss the paper; take notes and print out the paper (or bring a laptop).
Plan for Thursday:
PowerPoint slides: Introduction to the course
PowerPoint slides: Critical reading skills
The goals of stratigraphy are to improve our knowledge and understanding of Earth's rock bodies and their history.
Steno's Laws and a rock cycle were reviewed; we watched a portion of Earth Has A History.
Eustatic changes refer to changing global sea levels; isostatic changes refer to the vertical movement of continents.
A transgressive sequence forms as the shoreline moves landward from either submergence of the land or an increase in global sea levels. The lithologic units deposited as a result of eustatic changes follows Walther's Law (diachronous rock units).
Marker horizons, for example widespread volcanic ash deposits, provide a time reference where the entire unit is deposited at the same time.
PowerPoint slides: Stratigraphy
Tuesday: What is your marker of choice for a GSSP? Decide on Tuesday, or, if more time is needed, then Thursday is fine.
The demographic transition model was introduced (as was the suggestion of a universal basic wage). Read about Universal Basic Income in California.
See the following web sites:
Some sad news... "Hans Rosling, Swedish Doctor and Pop-Star Statistician, Dies at 68" from pancreatic cancer; see https://www.nytimes.com/2017/02/09/world/europe/hans-rosling-dead-statistician.html?_r=0
Watch some of Dr. Rosling's TED talks at https://www.ted.com/speakers/hans_rosling.
PowerPoint Slides: Population and exponential growth
Klein, G.D. (2015). The "Anthropocene": What is its Geological Utility? (Answer: It Has None!). Retrieved on 27 November 2016 from http://www.episodes.org/index.php/epi/article/view/79720/61837
[No geological basis for the Anthropocene; easy read]
Please click here for a copy of the assignment. Some general comments regarding the Lightning Round presentations:
Submit your assignment as a .ppt or .pptx file through Canvas by 9:00 a.m.
- Use the Assertion-Evidence model for PowerPoint presentations; links to this model may be found in the notes from Week 1 (above).
- Write a clear thesis statement (see the Purdue OWL page).
- Avoid cartoon images.
- Avoid unnecessary animation.
- Avoid bullets whenever possible.
- Avoid dark backgrounds (and avoid text that has similar colors as the background).
- Use cropping tool to modify images.
- Use a white box (or the snip tool) to cover extraneous information on images.
- Use large font (but do not yell), at least 28 point.
- Fit the text to the space and avoid orphans.
- Do not center-align text.
- While presenting, read what is on the screen.
- Practice your presentation (out loud).
One of the requirements for the Exploratory Paper is to cite current articles from two or three of the following news organizations: British Broadcasting Corporation World News, The Guardian, or The New York Times.
Finney, Stanley and Lucy Edwards, 2016. The "Anthropocene" Epoch: Scientific Decision or Political Statement? Retrieved on 27 November 2016 from http://www.geosociety.org/gsatoday/archive/26/3/pdf/i1052-5173-26-3-4.pdf
[Geological basis for the Anthropocene; moderate read; informative]
Linear growth occurs when a fixed amount is added at a fixed time (for example, a car traveling at a constant speed). The result is a straight line when plotted on arithmetic axes.
Exponential growth occurs when a percent of the total is added at a fixed time (for example, population). In this case, growth is proportional to the present size and thus results in a J-curve when plotted on arithmetic axes.
Doubling time is the time it takes for a value, that grows exponentially at a constant rate, to double in value. The equation for calculating doubling time is:
tD=ln(2)/λ
where:
tD is the time it takes for a quantity to double in value
λ is the growth rate
Please completed the attached assignment and bring a printout to class.
The mass of an atom, and ultimately the density of all materials, is controlled by the total number of protons and neutrons in the nucleus of an atom. We name the atom by counting the number of protons, and we name the isotope of an atom by adding the number of neutrons to the number of protons.
The size of the atom, and bonding atoms together, is primarily controlled by the electron cloud.
The weak nuclear force controls the mechanism and rate of decay of unstable isotopes. Unstable isotopes can be used for dating geological events, medical technology, nuclear energy, and nuclear weapons.Nuclear chemistry allows us to date geological events by use of unstable isotopes. Isotopes can be identified by use of a mass spectrometer. Unstable isotopes generate radiation as the nucleus undergoes spontaneous decay. The weak nuclear force controls the rate and mechanism of the decay of the nucleus.
Common decay mechanisms include:
Half-life is the average amount of time required for one half of the original number of radioactive atoms (parent atoms) to decay to child (daughter) products. Unstable isotopes are used for absolute dating. Each isotope has a defined half life. After five half lives, only 1/32 of the parent isotope remains; this small amount is difficult to measure accurately so we choose an isotope that has a half life appropriate to the age of the feature of interest (for example, depositional age, age of crystallization, or age of metamorphism).
PowerPoint Slides: Isotopes
Thursday: Lightning Round presentations


General comments from the lightning round presentations:
General comments from the exponential growth assignment:
The isotope of choice for nuclear power is 235U because it is easily fissile, yet 235U represents only 0.7204% of all uranium; 99.2742% of all uranium is 238U. Enriched uranium is represented by a concentration of 3-5% of 235U. Enriched uranium is used in nuclear power plants where the uranium splits (nuclear fission), gives off heat, and numerous daughter products that have long and varied decay chains. The decay chain for 238U is also long and varied that it spawns numerous unstable isotopes that give off radiation in perpetuity. The heat given off when splitting 235U is used to boil water, that turns to steam, that turns a turbine, that spins a generator, that makes electricity. Nuclear power is an expensive way to boil water.
Highly enriched uranium is represented by a concentration of 90-95% of 235U and is used in nuclear weapons. By concentrating 235U, that is enriching the sample with respect to 235U, what is left behind is referred to as depleted uranium (depleted with respect to 235U). Depleted uranium is used in armor-piercing bullets.
The Vermont Department of Health can also test your water for a large number of contaminants. A Gross Alpha test (section 4 RA) costs $45. Click for forms and ordering information; get the order form for all water tests (and costs); read the supplement associated with the order form.
Radon is an unstable isotope that occurs as a gas and forms from the decay of radium. Radon gas is a problem in many dwellings because radium may often be substituted for other elements that have two valence electrons. According to a report by the National Academy of Sciences, radon is estimated to cause between 15,000 and 22,000 lung cancer deaths per year. It is the second leading cause of lung cancer after smoking. Please read more about Radon found at the Vermont Health & The Environment website.
Read about a Russian spy who exposed Russian President Putin's ring of corruption and was subsequently poisoned by radioactive 210Po while in London. Or learn more about the "Radium Girls" who are still glowing in their coffins
Zalasiewicz et al. (2016) commented on the paper by Finney and Edwards (2016). See their comments at http://www.geosociety.org/gsatoday/comment-reply/27/2/GSATG309C.1.pdf (note that the et al. component represents 25 other authors).
Finney and Edwards (2016b) replied to Zalasiewicz et al. (2016) at http://www.geosociety.org/gsatoday/comment-reply/27/2/GSATG326Y.1.pdf.

Note: when grading these quizzes it was clear that not everyone read the paper. Please read the assigned materials before coming to class. In that way, the class discussions will be more lively and more beneficial.
The heat from the sun warms the surface of Earth. The warm surface radiates heat (long wave radiation — IR, infrared) back into the atmosphere. The long wave radiation interacts with molecules of a specific size and structure; these types of molecules are known as the greenhouse gases. The greenhouse gases (CO2, CH4, H2O, O3, NOx, and CFC) absorb the heat radiated from Earth and warm the atmosphere.
There are a lot of web sites related to global warming, see the greenhouse gas inventory, or the Global Climate Change Student Guide for more detailed information about climate change over space and time. See the Carbon Dioxide Information Analysis Center to find answers to questions relating to the atmospheric lifetime of carbon dioxide and methane, numerical estimates for sources and sinks of carbon, and greenhouse gas atmospheric residency times. See CO2Now.org for up-to-date, refereed, and clear information regarding climate change. See the NSF page for a video of carbon dioxide chemistry and function.
NASA reported that 2005 is the warmest year in over a century, later NOAA reported that Jan 2006 was the warmest January on record, later NASA reported that 2009 was the second warmest year on record, recently, March 2010 was reported as the warmest month on record, most recently 2012 was reported as the warmest year ever! Recently, it was reported that 2014 was the warmest year on record! In January 2017, NOAA reported that 2016 was the warmest year on record. What do you think the future holds?
Heat radiated from Earth is trapped by the greenhouse gases – the greenhouse gases are not trapped (however, gravity does hold Earth's atmosphere to the planet, so, in one sense, the gases are trapped by gravity). Heat is generated (radiated) by Earth's surface (only minimal energy is reflected off Earth's surface) and radiated into the atmosphere. Greenhouse gases heat up because they trap the energy radiated from Earth's surface. This trapping of the long wavelength energy results in an increase in the global average temperatures (commonly referred to as global warming). Greenhouse gases let energy from the sun warm Earth's surface, but trap the energy generated by Earth's surface, therefore warming the atmosphere.
Global average temperatures (15.0ºC or 59.0ºF) have risen approximately 0.7ºC in the past 100 years. Is the temperature rise a result of anthropogenic emissions of greenhouse gases, the Milankovitch cycles, global sunspots, volcanism, or something else? Increased global temperatures will result in more floods, more storms, more droughts, shifting climatic belts, and disruption to the food cycle. Could the warming be due to the sunspot cycle or (here)? Geologically rapid changes in Earth's atmosphere may indicate the onset of a new ice age; read about the potential for a new ice age because of global warming, by Woods Hole Oceanographic Institute. Read more about the discovery of rapid climate change. On 2 Dec 2003, two of the nation’s premier atmospheric scientists, after reviewing extensive research by their colleagues, say there is no longer any doubt that human activities are having measurable — and increasing — impacts on global climate. Read about the effects of Greenland's receding ice.
The Intergovernmental Panel on Climate Change (IPPC) has been established to assess scientific, technical and socio-economic information relevant for the understanding of climate change, its potential impacts and options for adaptation and mitigation. The IPCC Fifth Assessment Reports may be found here. The 45th Session of the IPCC, held 28 - 31 March 2017, took place in Guadalajara, Jalisco, Mexico; click here for the reports.
Ozone, O3, absorbs incoming ultraviolet (UV) wavelengths (short), and re-radiated longer-wavelength infrared (IR) heat from Earth. The ozone in the stratosphere protects organisms from the harmful UV radiation, yet is also a greenhouse gas that traps heat from Earth, and is a major component of smog in the troposphere. Chlorofluorocarbons (CFC), such as refrigerants, electronic parts cleaners, degreasers, and blowing agents, destroy ozone. Find out the UV index in Johnson today, see a daily contour map of the UV index for the US, or learn more about the science of ozone depletion. See the EPA's article on ozone: Good up high bad nearby. Read a recent report that describes a slightly smaller ozone hole.
There is no such thing as a safe tan. See the American Academy of Dermatology views on skin cancer and tanning booths. The Mayo Clinic recently reported a dramatic rise in skin cancer in young adults (and particularly in women in their 20's and 30's). Read about the Mayo Clinic's views on UV light. Review state laws regarding use of tanning booths, or a recent article in JAMA Dermatology. The University of Vermont Health Network just purchase a pulsed xenon UV disinfection machine that uses UV to sterilize rooms in the hospital; click here for the Xenex website.
Chlorofluorocarbons (CFC) are used as refrigerants, fire suppressors, degreasers and more. It takes about ten years for these synthetic CFCs to rise into the stratosphere, into the layer where ozone is concentrated, and destroy ozone molecules. Ozone protects life on Earth from harmful ultraviolet radiation. The ozone layer is slowly being depleted by CFCs. The Montreal Protocol of 1987 proposed the elimination of CFCs and replacement with hydrochlorofluorocarbons (HCFC). As a result of this action, the destruction of the ozone layer is slowing, yet it still continues. Unfortunately, however, HCFCs are potent greenhouse gases and thus help to accelerate global warming. We, the people, make choices that have global significance.
The greenhouse gases (CO2, CH4, H2O, O3, NOx, and CFC) absorb the heat radiated from Earth's surface and warms the atmosphere. Earth's surface is warmed by a wide range of wavelengths coming from the sun and passes through the atmosphere. Earth's warm surface radiates infrared (IR) radiation back into the atmosphere. The greenhouse gases are just the right molecular size to resonate (vibrate) with the infrared radiation and thus warms Earth's atmosphere. Greenhouse gases trap IR coming from Earth's surface.
The ozone layer is slowly being depleted by chlorofluorcarbons (CFCs). As the ozone layer thins, more ultraviolet (UV) passes through the atmosphere and results in skin cancer.
The depleting ozone layer is not causing global warming, and global warming is not a cause of the ozone problem.
Watch a visualization of how CO2 circulates in the atmosphere.
PowerPoint slides: Climate change
Spring break arrived early do to an overabundance of caution expressed by NVUJ regarding the COVID-19 virus. I do hope you are able to settle into your new surroundings.
The schedule, and some activities in this course, will be reorganized, so please keep checking back to this web page. For example, the second draft of the writing assignment is due before we return to campus. The writing assignment will be submitted as a .pdf file using Canvas. I am hoping we will be back on campus for the oral presentations.
All future assignments will be submitted through Canvas.
The Zoom link for this course can be found on Canvas, on the Announcements tab; recordings of all lectures can also be found on Canvas.
Please send me an email if you have any questions about this class, about NVUJ, or anything else that you think is appropriate. I will do whatever I can to make accommodations.
--------
Tuesday: i) review the exam, ii) discussion of climate change, and iii) discuss the final draft of the paper.
Thursday: Paper discussion (see below) and a quiz.
Thanks for pointing out that the key is incorrect.
--------
The average grade on the mid-term exam was 76.2% (range: 37.4—108.3). The grades have been posted on Canvas.
Waters, Colin N., Jan Zalasiewicz, Colin Summerhayes, Anthony D. Barnosky, Clement Poirier, Agnieszka Galuszka, Alejandro Cearreta, Matt Edgeworth, Erle C. Ellis, Michael Ellis, Catherine Jeandel, Reinhold Leinfelder, J. R. McNeill, Daniel deB. Richter, Will Steffen, James Syvitski, Davor Vidas, Michael Wagreich, Mark Williams, An Zhisheng, Jacques Grinevald, Eric Odada, Naomi Oreskes, and Alexander P. Wolfe (2016). The Anthropocene is Functionally and Stratigraphically Distinct From the Holocene. Science, v. 351(6269), p. 1-10.
Tuesday: i) discuss oral presentations and ii) global warming
The Tuesday lecture was recorded and can be played back here.
Thursday: Lewis and Maslin (2015) paper discussion.
The following document is available: Discussion paper review sheet. When submitting the review, convert the file to a .pdf file.
If you choose to submit a final draft of the paper, it is due on Thursday, 2 April 2020 (.pdf format only).
The following documents are available:
The heat from the sun warms the surface of Earth. The warm surface radiates heat (long wave radiation — IR, infrared) back into the atmosphere. The long wave radiation interacts with molecules of a specific size and structure; these types of molecules are known as the greenhouse gases. The greenhouse gases (CO2, CH4, H2O, O3, NOx, and CFC) absorb the heat radiated from Earth and warm the atmosphere.
There are a lot of web sites related to global warming, see a general article by NOAA or the greenhouse gas inventory. View the Global Climate Change Student Guide for more detailed information about climate change over space and time. See the Carbon Dioxide Information Analysis Center to find answers to questions relating to the atmospheric lifetime of carbon dioxide and methane, numerical estimates for sources and sinks of carbon, and greenhouse gas atmospheric residency times. NASA recently reported that 2005 is the warmest year in over a century, later NOAA reported that Jan 2006 was the warmest January on record, later NASA reported that 2009 was the second warmest year on record, recently, March 2010 was reported as the warmest month on record, most recently 2012 was reported as the warmest year ever! Recently, it was reported that 2014 was the warmest year on record! Now, 2016 appears to become the warmest year on record. What do you think the future holds?
Heat radiated from Earth is trapped by the greenhouse gases – the greenhouse gases are not trapped (however, gravity does hold Earth's atmosphere to the planet, so, in one sense, they are trapped by gravity). Heat is generated (radiated) by Earth's surface (only minimal energy is reflected off Earth's surface) and radiated into the atmosphere. Greenhouse gases heat up because they trap the energy radiated from Earth's surface. This trapping of the long wavelength energy results in an increase in the global average temperatures (commonly referred to as global warming). The greenhouse gases let energy from the sun warm Earth's surface but trap the energy generated by Earth's surface.Global average temperatures (15.0ºC or 59.0ºF) have risen approximately 0.7ºC in the past 100 years. Is the temperature rise a result of anthropogenic emissions of greenhouse gases, the Milankovitch cycles, global sunspots, volcanism, or something else? Increased global temperatures will result in more floods, more storms, more droughts, shifting climatic belts, and disruption to the food cycle. Could the warming be due to the sunspot cycle or (here)? Geologically rapid changes in Earth's atmosphere may indicate the onset of a new ice age; read about the potential for a new ice age because of global warming, by Woods Hole Oceanographic Institute. Read more about the discovery of rapid climate change. On 2 Dec 2003, two of the nation’s premier atmospheric scientists, after reviewing extensive research by their colleagues, say there is no longer any doubt that human activities are having measurable — and increasing — impacts on global climate. Read about the effects of Greenland's receding ice.
Tuesday: Continued discussion of global warming. See the PowerPoint slides here.
Thursday: Zalasiewicz et al. (2011) paper discussion.
The following document is available: Discussion paper review sheet. When submitting the review, convert the file to a .pdf file.
Tuesday: Discussion of sea level rise. See the PowerPoint slides here.
Earth's axis of rotation is currently 23º26'
to the plane of the ecliptic. The angle varies between 21.5º to
25.5º during a 23,000 year cycle. The rotational axis currently
points toward the North Star. Earth orbits the sun in an
elliptical fashion and is farthest from the sun during northern
hemisphere summers; distance from the sun plays a minor role in
average seasonal temperatures. The temperatures are primarily
controlled by the angle the sun's rays make with the surface of
earth. The Milankovitch
Cycles attempt to explain variation in
solar insolation throughout geological time (click here for more information).
Thursday: Zalasiewicz et al. (2015) paper discussion.
Tuesday: En-ROADS Climate Solutions Simulator
COVID-19, like other pandemics before, are very painful, and will change how people interact with others. Yet this pandemic, and many other problems, are related to the climate crisis. The climate crisis is the most enduring and pressing issue we face.
Learn about the climate crisis and the choices we have to make by running simulations. Illuminate the conversation with information. Model the situation to provide context and remove sloppy thinking, illogical thinking, and poor understanding of basic science.
Start with a simple question: What will happen if we electrify all cars? Look for positive and negative feedback loops.
Use En-ROADS Climate Interactive to make models of reality. The PowerPoint about this model can be found here.
A 12 April 2020 paper in Scientific American reports that methane levels have reached an all-time high (see also https://www.esrl.noaa.gov/gmd/ccgg/trends_ch4/).
A 16 April 2020 paper in Science reports a long-term mega-drought in the US is already under way (see also the BBC World Service).
A 8 April 2020 paper in Nature (or abstract) argued that entire ecosystems could abruptly go extinct in this decade as a response to the climate crisis (see a summary in Gizmodo).
A 16 April 2020 article in the New York Times describes the relaxation of rules regarding coal burning power plants and the additional release of toxic chemicals.
Oral presentations on Thursday.
Nicole, Lauren, David, and Rebecca presented.
All presentations must be submitted on 23 April 2020 via Canvas.
Every student must submit an evaluation of the presentations each day, for all presentations. Please use the Student Assessment of Presentations form (and submit it as a .pdf file). Submit the Thursday evaluation of presentations by 10:00 am Friday.
The climate crisis is upon us. Models are built to help understand the problem, and work toward solutions.
The climate modeling community uses representative concentration pathways (RCP) to model radiative forcing in watts/meter2; learn about RCPs here.
Scientists, economists, and demographers explore how societal choices will affect greenhouse gas emissions through shared socioeconomic pathways (SSP); learn more about SSPs here.
Oral presentations this week.
Tuesday: Maggie, Sierra, Ari, Matt, and Javon will present.
Thursday: Alex, Issac, Lean and Matt will present.
Every student must submit an evaluation of the presentations each day, for all presentations. Please use the Student Assessment of Presentations form (and submit it as a .pdf file). Submit the Tuesday evaluation of presentations by 10:00 am Wednesday; submit the Thursday evaluation presentations by 10:00 am Friday.
--------
Tuesday: Emily, Jacque, Naomi, Zach, and Jordyn will present.
Every student must submit an evaluation of the presentations each day, for all presentations. Please use the Student Assessment of Presentations form (and submit it as a .pdf file). Submit the Tuesday evaluation of presentations by 10:00 am Wednesday; submit the Thursday evaluation presentations by 10:00 am Friday.
Thursday: Politics, personal choices, and decision making.
--------
Part 1 of the final exam will be available under the 'Assignments' tab on Canvas starting at 5:00 PM on Tuesday, 12 May 2020. It is due at 9:00 AM on Thursday, 14 May 2020.
Part 2 of the final exam will be availble under the 'Assignments' tab on Canvas starting at 9:00 AM on Thursday, and it is due one hour later at 10:00 AM.
The guidelines for the final exam are available here and now.
Good luck on all of your finals and thank you for participating in this class.
Watch Planet of the Humans for yet another perspective on the green movement, human nature, and the choices we must take.
We will not have time to discuss this film in class, nor will it show up on any exam or paper or project required for this course. Yet, I would be happy to speak with you about it outside of class, at any time.