Ozone is an interesting molecule because it absorbs infrared (IR) radiation (heat) emitted from Earth’s surface, and ultraviolet (UV) radiation from the sun. It is regarded as a greenhouse gas because it absorbs heat. It is the only greenhouse gas that also absorbs incoming UV radiation and therefore protects us from the harmful UV rays that cause skin cancer.
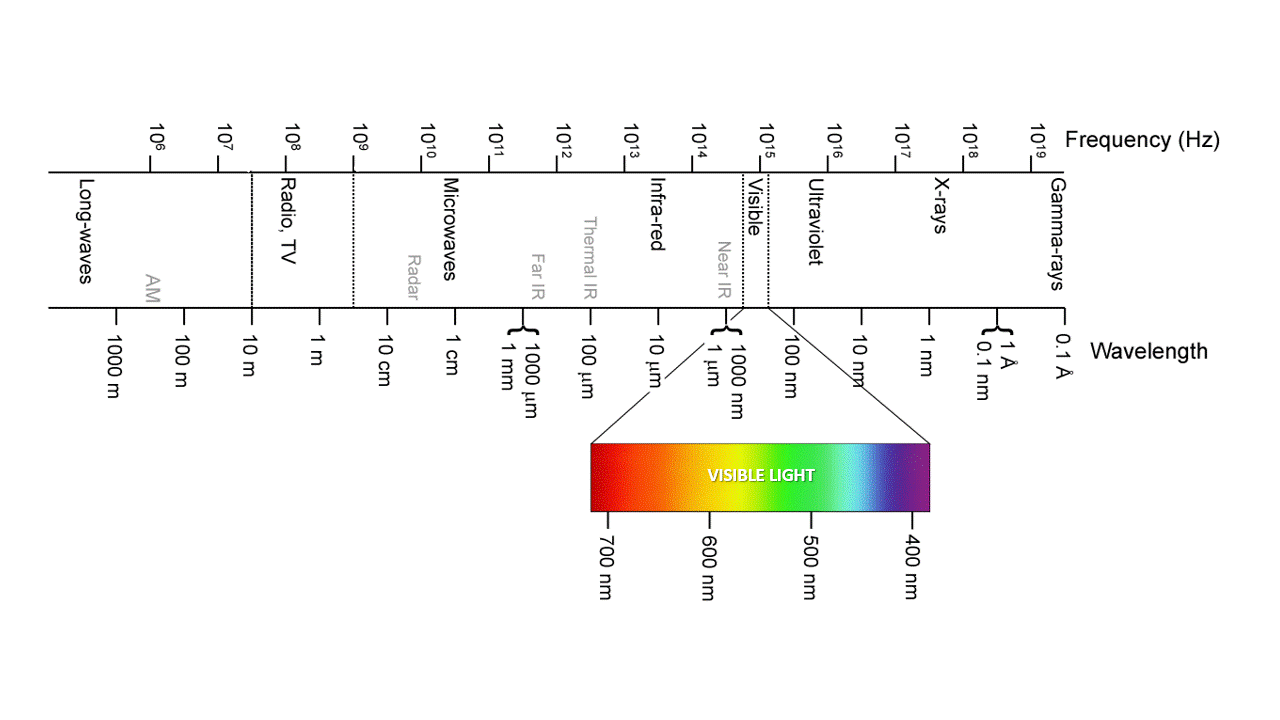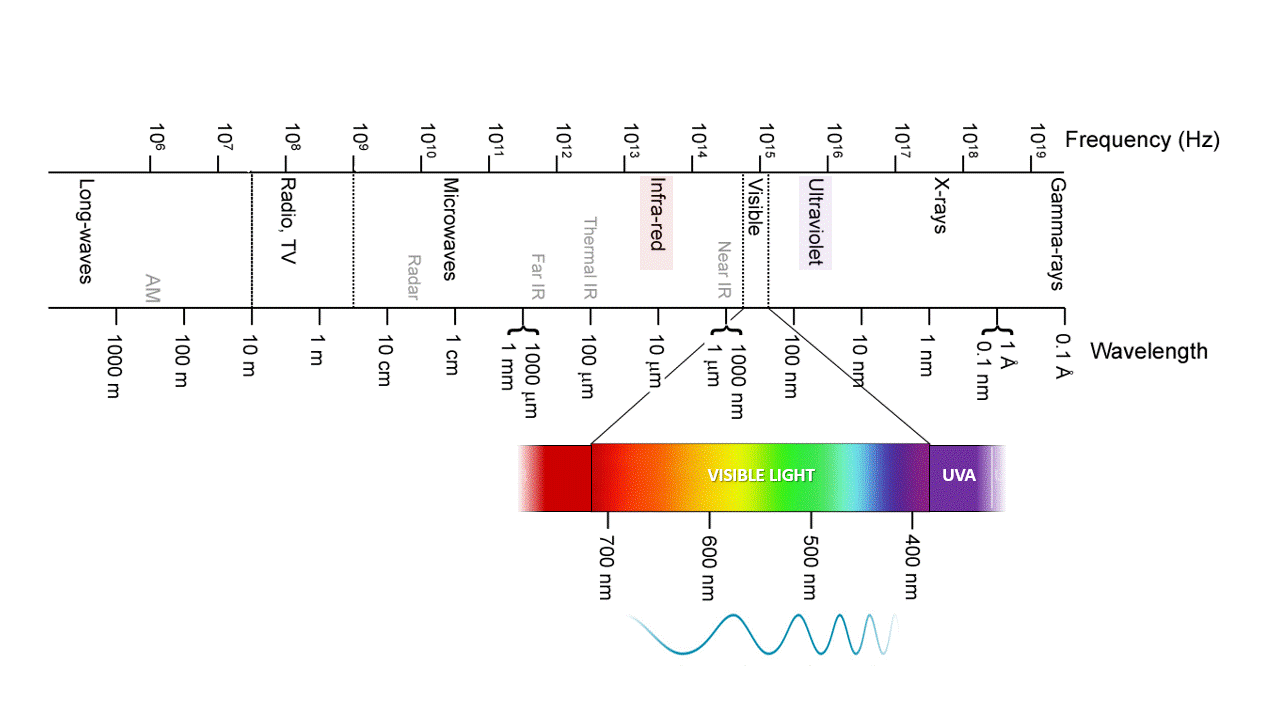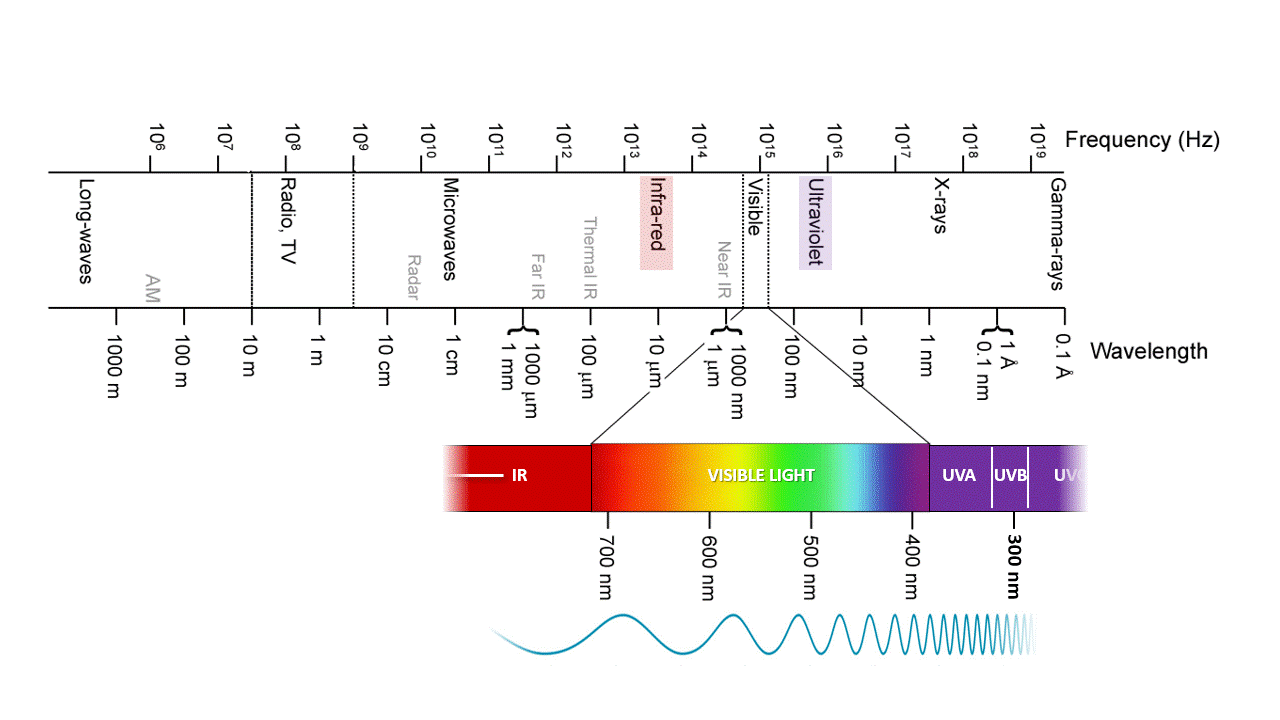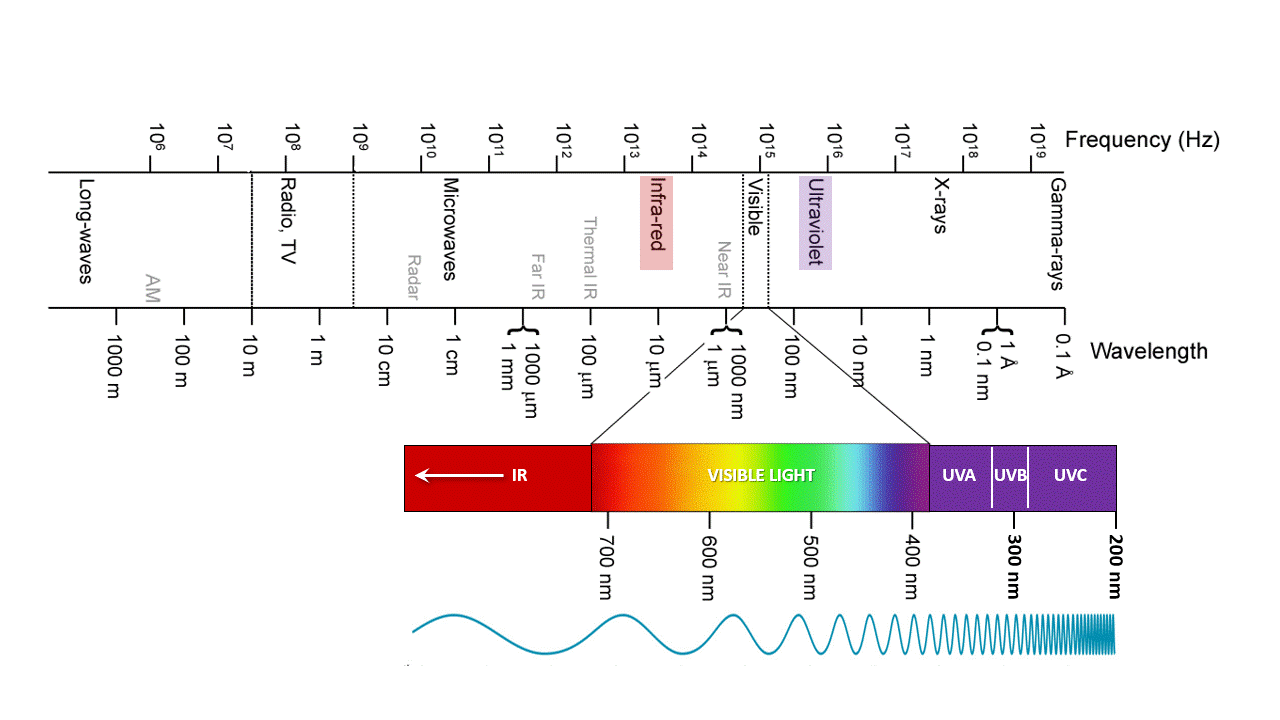
Ozone, O3, absorbs IR and UV because is just the right molecular size. UVC (100-280 nm) is the most damaging type of UV radiation, yet most of it is absorbed by atmospheric ozone. UVB (280-315 nm) is responsible for delayed tanning, burning, and skin cancers. UVA (315-400 nm) is the most common UV wavelength reaching Earth’s surface and is responsible for the tanning; it also promotes skin ageing and skin cancers. As the ozone layer becomes thinner, more ultraviolet light makes its way to Earth’s surface. It is best to stay covered up when the sun is out.
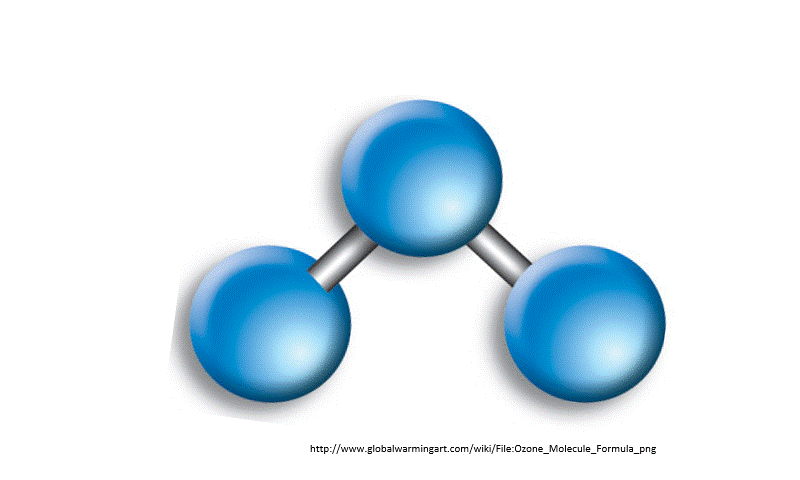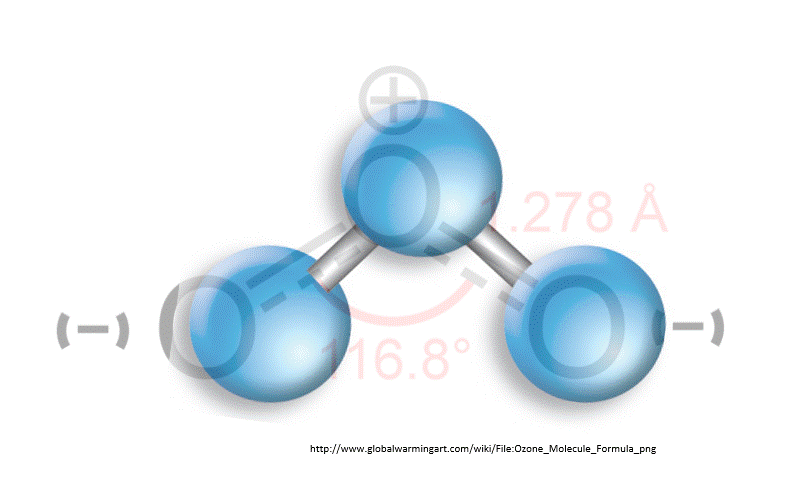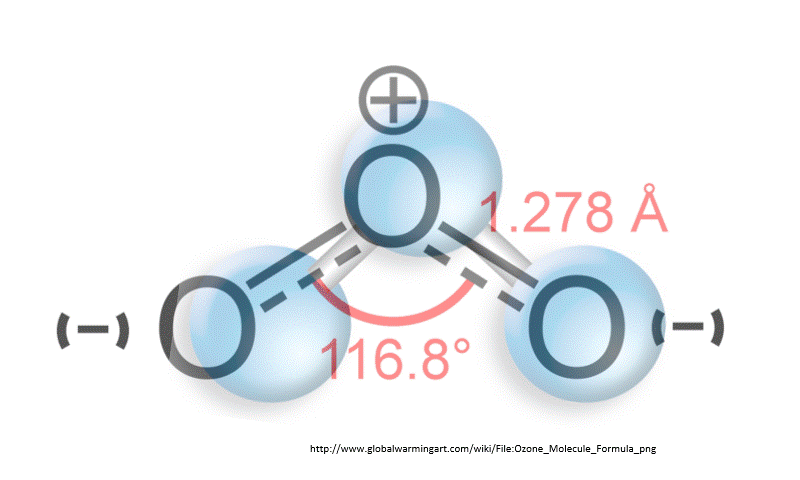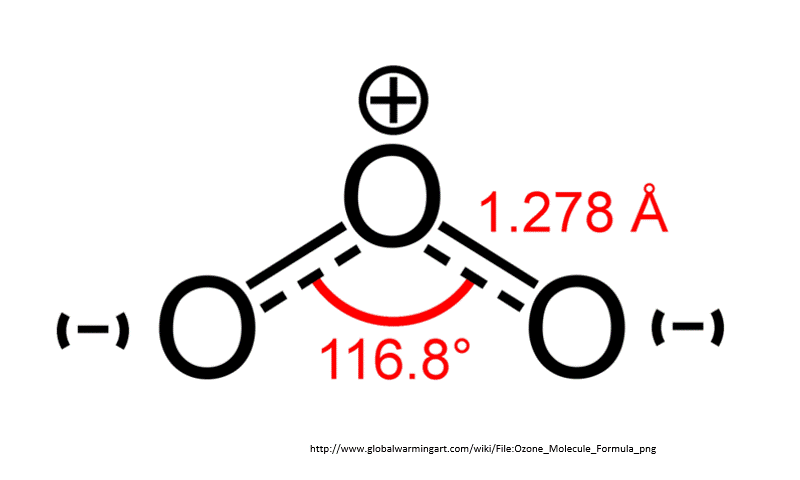
Chlorofluorocarbons (CFC) are synthetic molecules used in firefighting equipment, coolants in refrigerators and air conditioner, and as degreasers. These use CFC molecules are also, unfortunately, a greenhouse gas. That is, CFCs absorb heat emitted from Earth’s surface and have some responsibility for global warming. Worse, however, is that CFCs destroy ozone. A reduction in ozone allows more harmful UV rays to pass through Earth’s atmosphere.
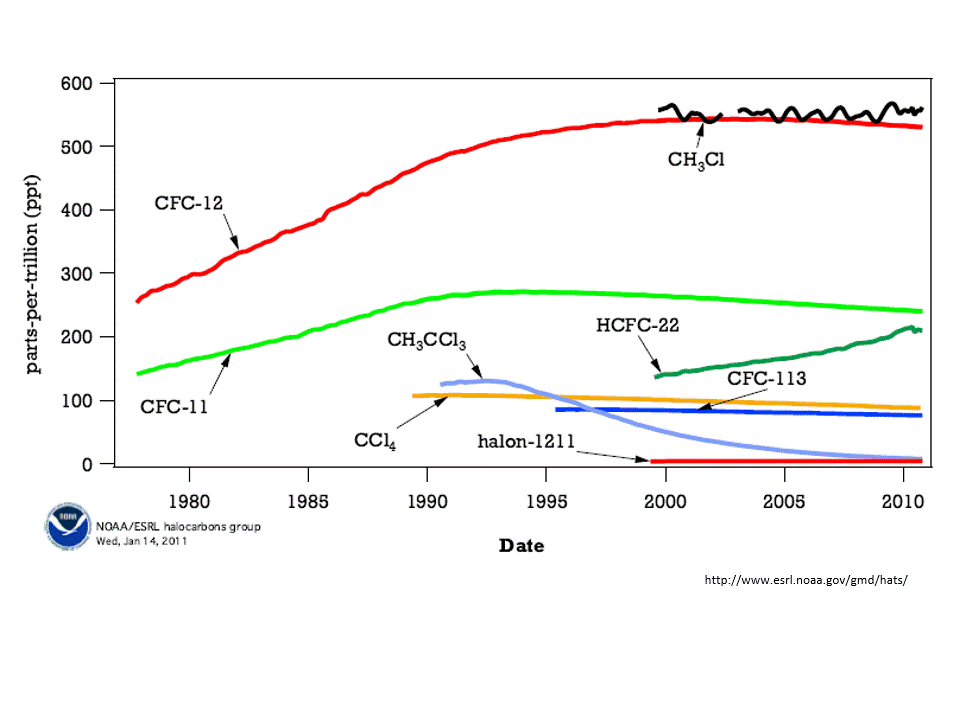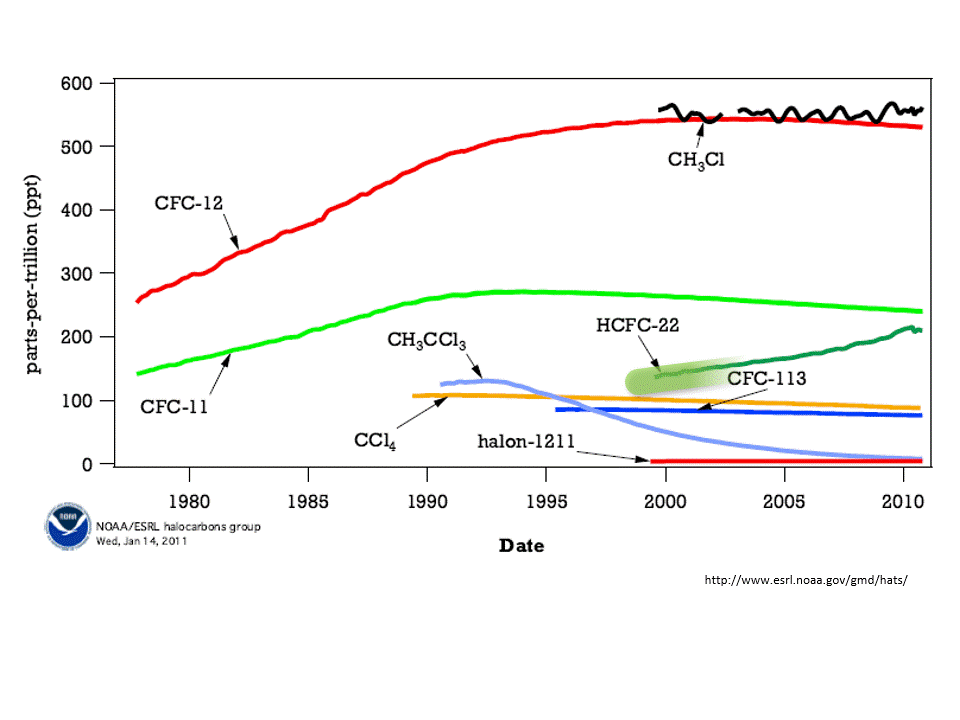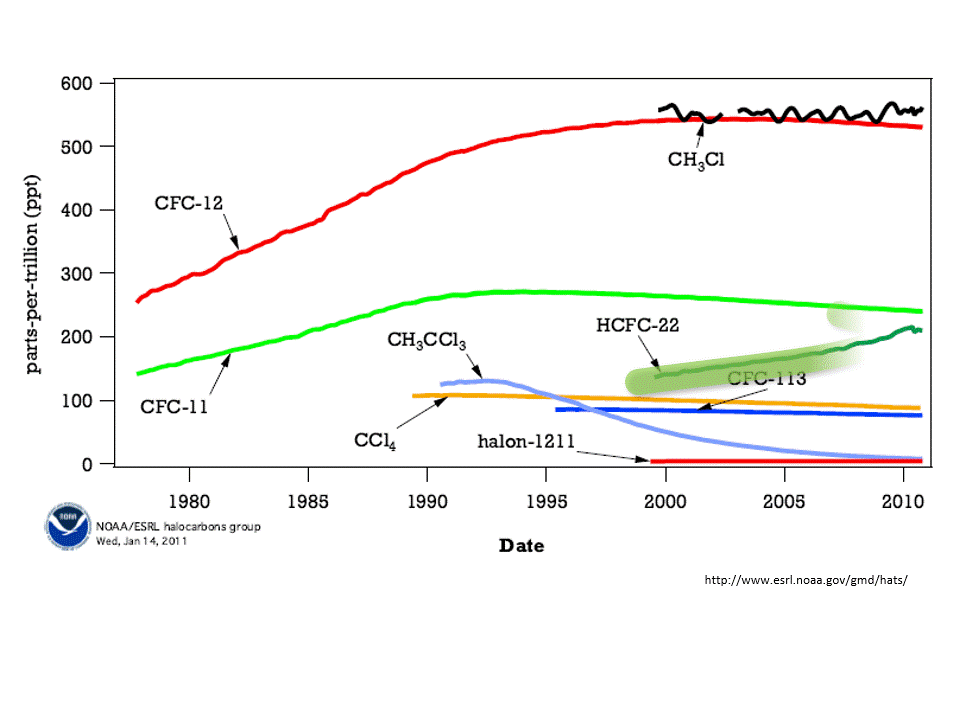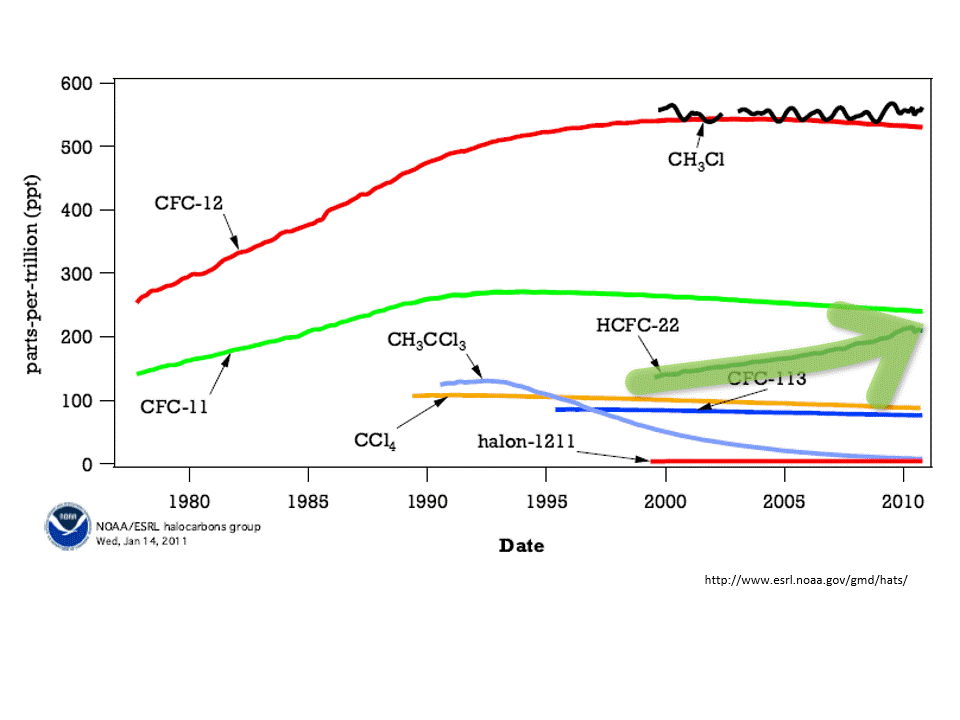
The concentration of atmospheric ozone is reduced in cold temperatures — that is why the thinning of the ozone layer was first noticed over the polar regions. When the atmospheric concentration of ozone is high, then the incoming harmful ultraviolet rays are reduced.
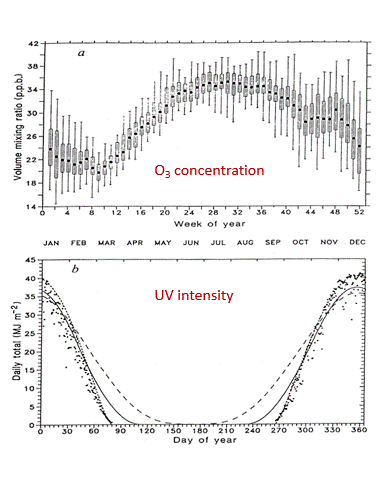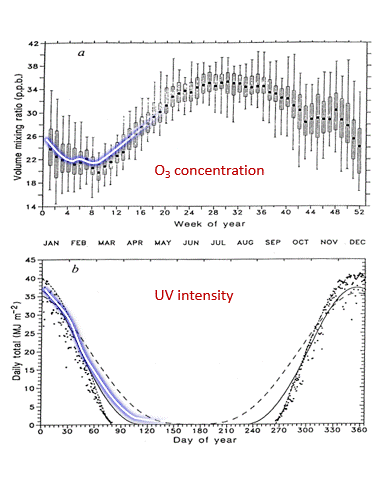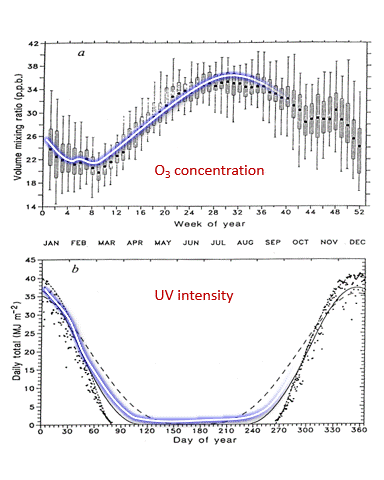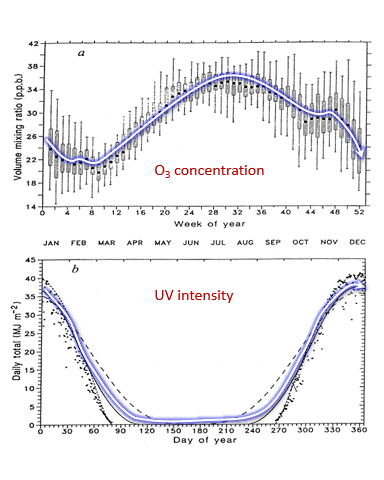

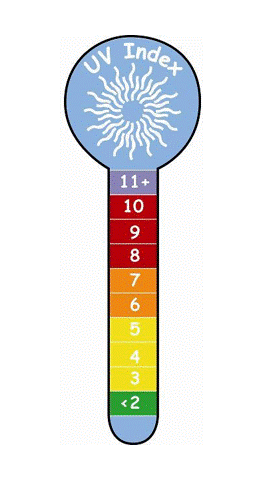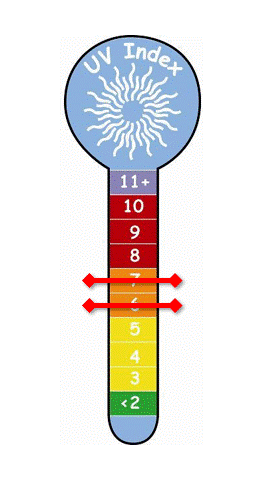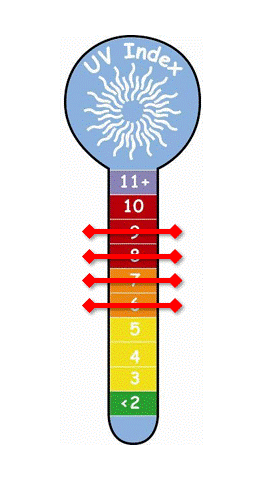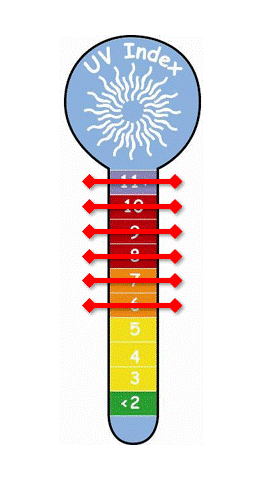
The ultraviolet index is a good measure of when it is safe to be outside. When the ultraviolet index is less than two, then sunscreen is not necessary. Check the local ultraviolet index every day before going outside and take appropriate actions.