by
Aaron Guilfoyle

Dioxin is a chemical family that is the by-product of common industrial practices. Dioxins are formed through the combustion of waste or fuels containing chlorine. Dioxin is also the byproduct of the chemical penta and is formed in the paper making process. The EPA considers dioxin to be one of the more deadly chemicals created by humans. Studies have shown that dioxin causes a number reproductive, developmental, and neurological problems in infants and children. Exposure has also been linked to certain forms of cancer. As Americans we are at a large risk because dioxin is found in many common food sources through the country. The common practice of waste combustion must be addressed; according to the EPA combustion of waste is the single highest imputes of dioxin into or environment.
Dioxin is the name given to a group of chemicals that is an unintentional byproduct of many common industrial processes. It is formed from use of chlorine when making paper, or when products containing PVC are burned. Dioxin has been found to be carcinogenic at very low levels of exposure, yet many foods we consume today contain dangerously high levels of dioxins. There is a possibility that dioxins are leading to the rise of medical problems worldwide. At this point in time it appears that dioxin pollution may be one of the more dangerous problems we are facing.
Dioxins are considered to be some of the most toxic chemicals known. This chemical which is a by-product of industrial processes that contain chlorine, is known to cause many human health problems. Dioxin is a known cancer-causing agent and at lower levels can cause reproductive system problems (EPA, 2001.
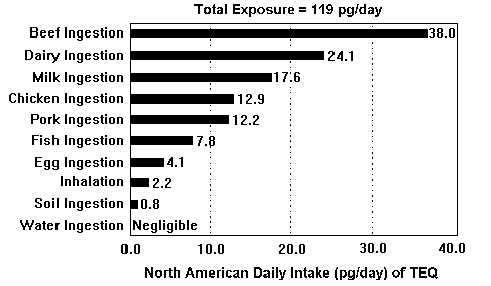
Dioxin is a name given to hundreds of chemicals. Not all are very dangerous but a few are very destructive. The EPA has found the acceptable level of dioxin exposure to be .006 pictograms per day (EPA, 2001). Studies have found however that much of the population of the US has already unacceptable levels of exposure to dioxin. A single McDonald's hamburger purchased in the US, contains 250 times what is considered to be an "acceptable daily dose" (Campbell and Ewall, 2001). Food products that come from animals have the highest levels of dioxin found in them. Figure 1 shows possible daily sources of dioxins.
Dioxins act much like the pesticide DDT; these chemicals accumulate in the fat cells of animals that are exposed to it. This means that in higher consumers, including humans, dioxin easily reaches toxic levels. Dioxins have a high resistance to degradation there for can travel over long distances and stay toxic in the environment for a long period of time (Europa, 2000).
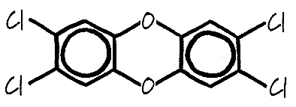
Dioxin is a shorter version of the name 2,3,7,8-tetrachlorodibenzo-p-dioxin, which was given to a group of chlorinated organic compounds that also contain carbon and oxygen (Shadoff, 2001). Figure 2 shows the basic structure of the dioxin molecule.

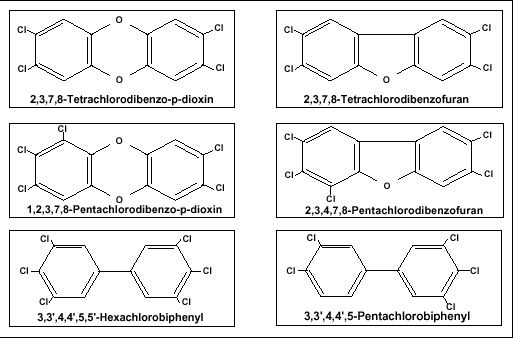
Dioxins are a member of a family of similar human made organic compounds. Dioxin is by far the most toxic. In fact, dioxin is considered to be the most toxic human made organic substances on the Earth, only radioactive waste is considered to be more dangerous (Campbell, 1999). There is very little difference between dioxin and many other compounds. Figure 3 shows the dioxin molecule compared to some of the other chemicals in its family.
Dioxin is an unintentional byproduct of many industrial processes. The first use of dioxin was during the Vietnam War. Dioxin was one of the deadly chemicals found in Agent Orange (Shadoff, 2001). A mixture of Agent Orange and kerosene was used as a defoliant during the conflict. The dioxin level that was found in the herbicide was not uniform. The range of content varied from one part per trillion too as high as sixty parts per million (Shadoff, 2001).
The largest source of dioxin comes from incinerators that burn municipal wastes (Figure 4). Much of the waste contains both chlorine and carbon, two essentials for the formation of dioxin. Combustion of fuel that contains both chlorine and carbon can also produce a number of different dioxin isomers. It is believed that lower temperature combustion such as wood burning produces more dioxins then a high temperature combustion process like burning coal (Shadoff, 2001).
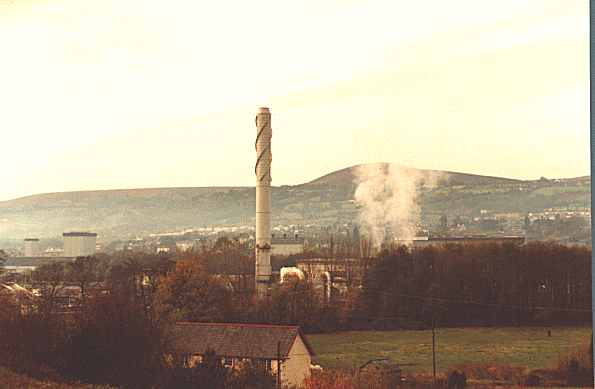
In a solid waste incinerator the break down of trace metals, chlorinated compounds, and organic materials takes places. As the gasses produced leave the main chamber, then tend to start to cool down from 1000oC. As this cooling occurs the gasses condense and molecular rearrangement takes place. This is when dioxin is formed. The proper temperature range for dioxin formation is between 300o to 650 o C. The ideal temperature is down around 300o C, this is when the most dioxin is formed (Lehman ET al., 1999).
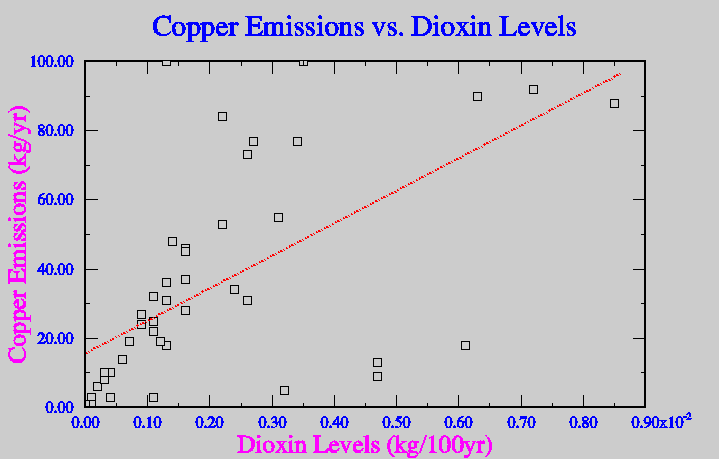
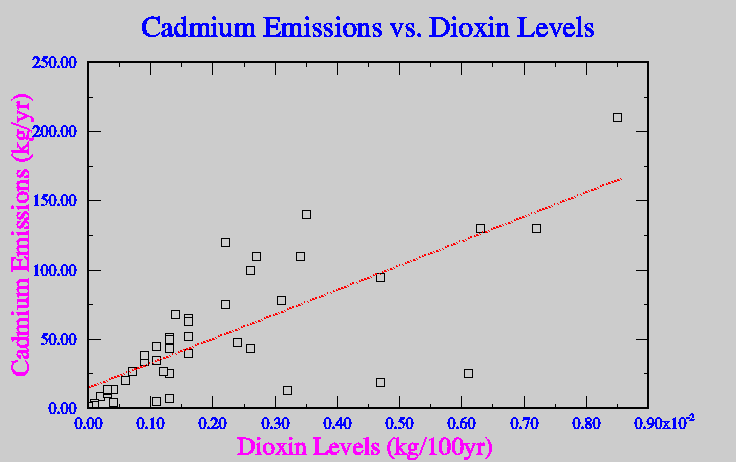
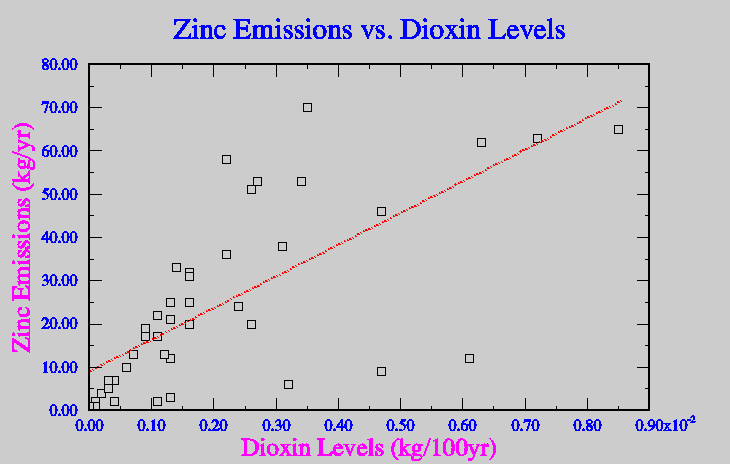
In attempts to avoid mass production of dioxin, municipal waste incinerators have installed spray dryers and scrubbers to try and instantaneously cool the emitted gasses, in ideal situations this avoids the production of dioxins all together. Unfortunately if there is a catalyst available even higher concentrations of dioxin can be released. A catalyst, which is in form of a heavy metal, can greatly increase the levels of dioxin produced while gasses cool in the spray dyer. Metals like copper, zinc and cadmium all will increase levels of dioxin emission if present (Lehman, 1999). Figure five show the correlation between the levels of production of dioxin and some similar heavy metals.
About 95% of all dioxins found in the atmosphere come from industrial processes that involve the burning of chlorinated products (Shadoff, 2001). Figure 6 below is a set of tables that shows USEPA estimated inventory for dioxin sources in the Untied States during 1995. The other five percent comes from sources like papermaking, pesticides, and wood treatments. The chemical Pentachlorophenol is a large source of dioxins. It is sometimes called Penta for short. Penta is used to treat commercial products like telephone poles, fence posts and lumber. The wood is dipped in a solution to make them waterproof. This common industrial chemical contains almost every known isomer of dioxin. While treating wood with this chemical workers often come in contact with the solution it self or with the treated wood. Studies shown this may possibly be the most common way that humans are exposed to dioxin through non-combustion processes (Shadoff, 2001).
| air: | (gm teq/year) | ||
| municipal waste incineration | 1,100 | secondary aluminum smelting | 17 |
| secondary copper smelting | 541 | oil combustion- industrial/utility | 9.3 |
| medical waste incineration | 477 | sewage sludge incineration | 6 |
| forest, brush, and straw fires | 208 | hazerdous waste incineration | 5.7 |
| cement kilns (hazardous waste burning) | 153 | vehicle fuel combustion -unleaded | 6.3 |
| coal combustion | 72.8 | kraft recovery boilers | 2.3 |
| wood combustion- residential | 62.8 | secondary lead smelters | 1.63 |
| wood combustion- indusrial | 29.1 | cigarette combustion | 0.81 |
| vehicle fuel combustion- diesel | 33.5 | boilers/industrial furnaces | 0.38 |
| cement kilns (non hazerdous waste burning) | 17.8 | crematoria | 0.24 |
| total | 2,745 |
| products: | (gm teq/year) | ||
| pentachlorophenol- treated wood | 25,000 | 2,4-dichlorophenoxy acetic acid | 18.4 |
| bleached chemical wood pulp and paper mills | 24.1 | non-incinerated municipal sludge | 7 |
| dioxazine dyes and pigments | 0.36 | total | 25,050 |
| land: | (gm teq/year) | ||
| non-incinerated municipal sludge | 207 | bleached chemical wood pulp and paper mills | 1.4 |
| total | 208.4 |
| water: | (gm teq/year) | ||
| bleached chemical wood pulp and paper millls | 19.5 | ||
| total | 19.5 |
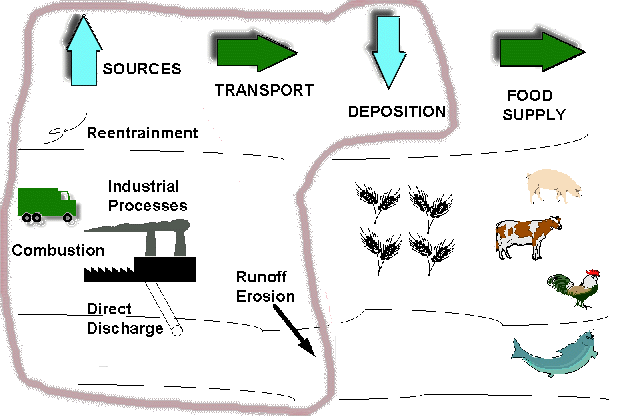
Human beings are exposed to dioxin simply by eating. Safely 90%, but possibly as much as 96% of all human exposure to dioxin is through a food source (CHEJ, 2001). How the dioxin gets to our food is quite simple. As was stated before the largest source of dioxin poisoning in the air is from the burning of waste and fuels. The water and land are both polluted from the runoff of soils polluted by penta treated wood, and from direct sources of discharge like papermaking. Wide spread deposition also occurs on both land and water as the dioxins released by combustion processes falls back to the earth (EPA, 2000). The flow of dioxin through the environment occurs through certain transport mechanisms. Dioxin is moved over long distances by the wind. The chemical can be leeched out of contaminated soils and travel long distances without breaking down. Wind erosion of soils can pick up and move dioxin in the form of dust particles. (EPA, 2000).
Dioxin is considered to be a persistent organic pollutant. "Compounds that travel through thousands of miles, accumulate in the food chain, and persist in the environment, taking up to centuries to fully degrade." (Fisher, 1999) These chemicals tend to resist photolytic, biological, and chemical degradation. They are semi volatile and therefor have the ability to move long distances before deposition. Due to their nature dioxins move in what is referred to as the grasshopper effect. This is a process by which POP's move long distances from their source in the air through a repeated pattern of release and redeposition (Fisher, 1999). By this process dioxin and related chemicals have been able to spread all over the world.
Dioxin can be found in a number of reservoirs and sinks. The build up in soils and sediments can be released later. The same is true for products that contain dioxin. The chemical can become trapped for long periods of time in deep undisturbed sediments. One of the most important storages of dioxin is with in a common property of halogenated compounds. Dioxin has very little affinity to dissolve in water, however it will readily absorb and dissolve into lipids. This means that animals store dioxin in their fat cells (Fisher, 1999). Considering these characteristics and the pathways of travel and deposition for dioxin that was discussed above, Figure 7 below illustrates the flow of dioxin through our environment.
The EPA considers dioxin to be one of the most toxic chemicals that they deal with (Johnson, 1997). Recent studies and findings lately have made them to reevaluate their stance on dioxin. Recently new studies have found that health effects associated with dioxin are connected to a specific protein. This protein is necessary to carry the dioxin into a human's tissue. The protein is known as the Ah receptor. The presence of this receptor and certain background levels of dioxin in specific organs lead to a wide variety of human health problems (Johnson, 1997).
Studies in Holland have found a number of health effects associated with normal background levels of exposure to dioxin in prenatal infants (CHEJ, 2001). These effects include:
The same study in Holland concluded that it was not possible to trace these developmental problems found at 18 months if gone undetected until age 42 months. At this stage it is no longer possible to link the problems to dioxin. This means if these problems go undetected early on then the cause may not be found. However they did find that it was possible to trace a drop in play activities and a rise in depression due to prenatal exposure (CHEJ, 2001).
Studies also showed different health problems that are associated to normal levels of daily exposure to dioxin to young infants (CHEJ, 2001). These health effects include:
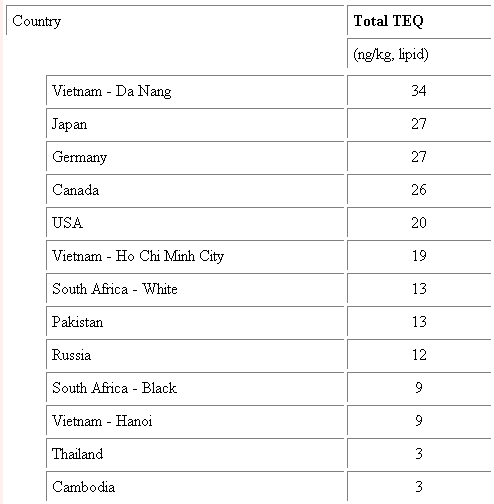
It is very common for young infants to be exposed to dioxin. Like many deadly chemicals dioxin accumulates in the fat cells of animals. This means that women who are producing milk pass dioxin onto their babies through normal feeding. Dioxin is found in samples of breast milk through out the entire world. Figure 8 illustrates dioxin levels found in breast milk from various countries around the world.
Further studies have shown that there are a number of other effects associated with the exposure to dioxin in human beings. It has been found that dioxin is linked to hypo-mineralization defects in permanent teeth. It appeared at first that this only occurred in babies that were breast fed, but more data found that the problem was also present in other infants as well. Studies on US children found that exposure to normal daily doses of dioxin can lead to defects in the development of human neurological development as well as reproductive development (CHEJ, 2001).
The effects of dioxin exposure on adults have also found to be significant. The major findings include decreased sperm counts in men and higher risk of diabetes in all humans. In the case of sperm count, workers who were studied were found to have lower than normal levels of testosterone and higher then normal levels of follicle-stimulating and luteinizing hormone levels, both of these problems can reduce sperm count (CHEJ, 2001). Dioxin interferes with the hormone insulin and can alter glucose tolerance leading to diabetes. A study showed that workers exposed to dioxin had a 50% chance of developing diabetic symptoms after ten years (CHEJ, 2001).
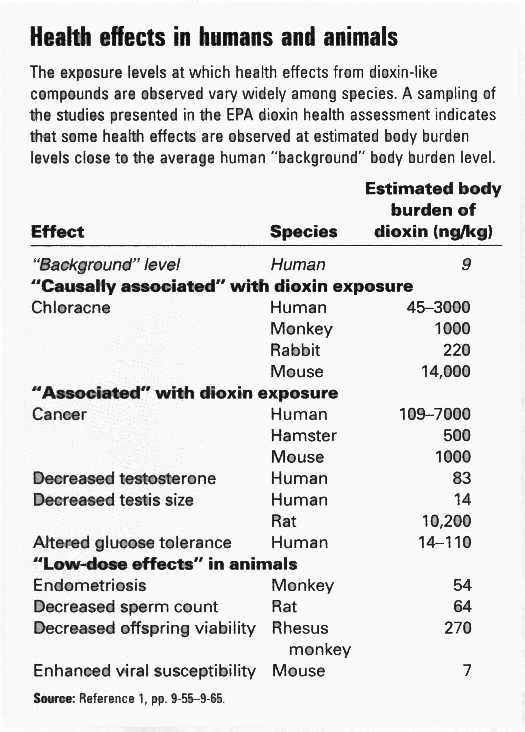
One of the reasons that dioxin is considered to be so toxic is because of its carcinogenic effects at relatively low levels of exposure (EPA, 2001). Figure 9 is a chart of levels of dioxin exposure and the associated health problems for both animals and humans. Due to limitations on experiments, we can not experiment on humans; we can only estimate what levels of exposure will lead to cancer from data collected during animal studies. Whether or not we are able to quantify the exact level of exposure or not the fact remains dioxin causes cancer in humans and animals alike (CHEJ, 2001). Studies on factory workers in both Holland and Germany have been able to conclude this with out a doubt.
According to the Center for Health and Environmental Justice, "American people are at serious risk from their daily intake of dioxin in food." Below is a summary that encompasses new studies and current information know about dioxin written by the Center for Health and Environmental Justice (CHEJ, 2001).
In conclusion dioxin poisoning is becoming a major problem in the US. Many of our everyday practices are leading to the release of large amounts of dioxin into our environment. As a culture we must take the time to understand just how significant the level of pollution is. Then we have to take steps to prevent further dioxin pollution. As the deaths contributed to cancer grow, a finger must be pointed in the way of dioxin and the forms of industry that lead to its release. We can not allow this problem to go the way of the fossil fuel argument. The need for alternative energy has been on going for years, yet there has been no incentive for change. Industry must not be aloud to control the amount of dioxin that they release. As of right now the US has one of the strictest standards for dioxin in the industrialized world. Many people would like to see this changed. As a culture we must stop the continual poisoning of our population by chemicals like dioxin.
If you are not scared of the dioxin problem that is arising, you should be. Preventing the creation of dioxin is not the easiest thing to do. Personally we can take steps by not burning trash in our back yards. One can also purchase unbleached paper when possible. Not using penta treated wood would help to prevent dioxin release from pressure treated lumber.
One of the most effective ways to help solve our dioxin problem is through letter writing. Writing letters to elected officials can help to shape legislation towards stopping mass releases of dioxin. Asking the government to stop burning municipal and medical waste would be a good step. We must also find a way to force industry to find an alternative to the chemical penta. Below are some different form letters that you can download and send to the appropriate parties, you will also find a list of addresses to use or a sight to locate the correct address.