Slide 13 of 44
Notes:
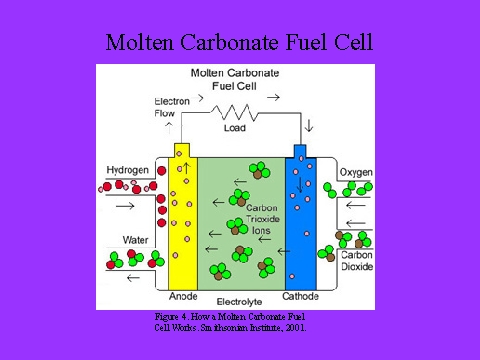
Molten carbonate fuel cell
- They use a molten carbonate salt mixture as its electrolyte.
- The electrolyte usually consists of lithium carbonate or potassium carbonate. The molten carbonate
- At the anode, there is a reaction between hydrogen and carbonate ions from the electrolyte which produces The salt mixture is liquid and is a good ionic conductor.
- water and carbon dioxide while releasing electrons to the anode.
- At the cathode oxygen and carbon dioxide are combined from the oxidant stream with electrons from the cathode to produce carbonate ions which enter the electrolyte.
Molten Carbonate fuel cells (MCFC) use high-temperature compounds of salt (like sodium or magnesium) carbonates (chemically, CO3) as the electrolyte. Efficiency ranges from 60 to 80 percent, and operating temperature is about 650 degrees C (1,200 degrees F). Units with output up to 2 megawatts (MW) have been constructed, and designs exist for units up to 100 MW. The high temperature limits damage from carbon monoxide "poisoning" of the cell and waste heat can be recycled to make additional electricity. Their nickel electrode-catalysts are inexpensive compared to the platinum used in other cells. But the high temperature also limits the materials and safe uses of MCFCs—they would probably be too hot for home use. Also, carbonate ions from the electrolyte are used up in the reactions, making it necessary to inject carbon dioxide to compensate