Slide 11 of 44
Notes:
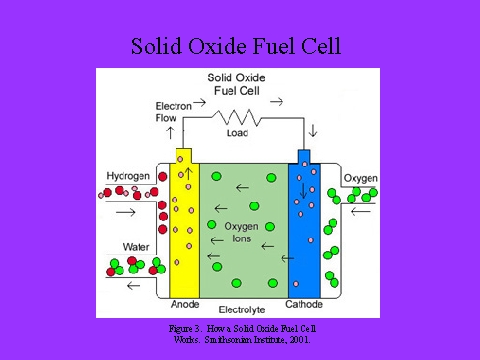
- Solid oxide F.Cs uses a ceramic, solid phase electrolyte.
- In operation, hydrogen or carbon monoxide (CO) in the fuel stream reacts with oxide ions (O=) from the electrolyte to produce water or CO2 and to deposit electrons into the anode.
- The electrons pass outside the fuel cell, through the load, and back to the cathode where oxygen from air receives the electrons and is converted into oxide ions which are injected into the electrolyte.
- It is significant that the SOFC can use CO as well as hydrogen as its direct fuel.