Slide 9 of 44
Notes:
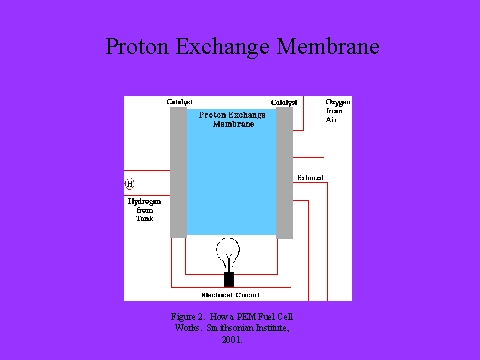
- The proton exchange membrane is a thin plastic sheet that allows hydrogen ions to pass through it.
1. At the anode the hydrogen molecules give up electrons and form hydrogen ions, a process which is made possible by the platinum catalyst.�
- The electrons travel to the cathode through an external circuit, producing electrical current. This current can perform useful work by powering any electrical device (such as an electric motor).�
- The proton exchange membrane allows protons to flow through, but stops electrons from passing through it. As a result, while the electrons flow through an external circuit, the hydrogen ions flow directly through the proton exchange membrane to the cathode, where they combine with oxygen molecules and the electrons to form water.�
- In this way, hydrogen fuel's natural tendency to oxidize and form water is utilized to produce electricity and useful work.�
- No pollution is produced and the only resulting products are water and heat.��