Slide 4 of 44
Notes:
Fuel cells produce energy in the form of electricity and heat as long as fuel is supplied.
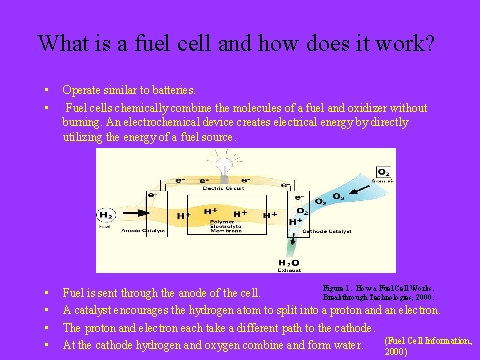
A fuel cell consists of two electrodes sandwiched around an electrolyte. Oxygen passes over one electrode and hydrogen over the other, generating electricity, water and heat. Hydrogen fuel is fed into the “anode” of the fuel cell. Oxygen enters the fuel cell through the cathode. Encouraged by a catalyst, the hydrogen atom splits into a proton and an electron, which take different paths to the cathode. The proton passes through the electrolyte. While the electrons create a separate current that can be utilized before they return to the cathode, to be reunited with the hydrogen and oxygen in a water molecule.
A fuel cell system can utilize the hydrogen from any hydrocarbon fuel. Since a fuel cell relies on chemistry and not combustion, emissions from this type of a system would still be much smaller than emissions from the cleanest fuel combustion processes.
Fuel cells are only energy conversion devices. They cannot be separated—either conceptually or physically—from the infrastructure that delivers fuel. Any fuel cell system can provide power only after a series of infrastructure elements delivers energy from a primary energy source.